A theoretical study on hydrated sodium ion–phenylalanine clusters Na+(Phe)(H2O)n (n = 0–6; Phe = phenylalanine)†
Abstract
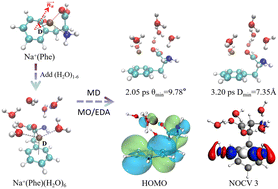
The cation–π interaction is of importance in many chemical and biological processes such as those involving protein geometries and functionals and ion channels. In this study, to understand the cation–π interaction between essential ions and protein in the water-aqueous environment, geometries, electronic structures, bonding properties, and dynamic stabilities of hydrated Na+–phenylalanine clusters Na+(Phe)(H2O)n (n = 0–6) were studied using density functional theory calculations and ab initio molecular dynamics simulations. After the addition of water molecules, Na+(Phe)(H2O)n structures change from a tridentate complex to quadridentate or pentadentate complexes while the cation–π interaction always exists. The fluctuation between quadridentate and pentadentate complexes results from the competition between cation–O bonding and hydrogen bonding. The charge analysis reveals that the positive charge is mainly located on the Na ion, whereas the further addition of water reduces the binding energy of water, electron affinity, and ionization potential. As the number of water molecules increases, the bonding interactions between the sodium ion and the remaining phenylalanine–water complex increase and correlate with the coordination number, in which the electrostatic interaction contributes more than the orbital interaction. The important orbital interaction terms come from the donation of the carboxyl and amino groups and water to the Na+ ion. Molecular dynamic simulations revealed that Na+(Phe)(H2O)6 is stable at 300 K.


 Please wait while we load your content...
Please wait while we load your content...