High-concentration LiPF6/sulfone electrolytes: structure, transport properties, and battery application†
Abstract
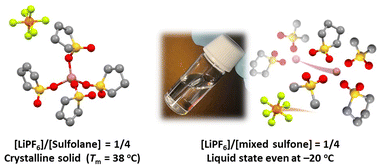
Non-flammable and oxidatively stable sulfones are promising electrolyte solvents for thermally stable high-voltage Li batteries. In addition, sulfolane-based high-concentration electrolytes (HCEs) show high Li+ ion transference numbers. However, LiPF6 has not yet been investigated as the main salt in sulfone-based HCEs for Li batteries. In this study, we investigated the phase behaviors, solvate structures, and transport properties of binary and ternary mixtures of LiPF6 and the following sulfone solvents: sulfolane (SL), dimethyl sulfone (DMS), ethyl methyl sulfone (EMS), and 3-methyl sulfolane (MSL). The stable crystalline solvates Li(SL)4PF6 and Li(DMS)2.5PF6 with high melting points were formed in the LiPF6/SL and LiPF6/DMS mixtures, respectively. In contrast, LiPF6/EMS, LiPF6/MSL, and LiPF6/SL/another sulfone mixtures remained liquids over a wide temperature range. Raman spectroscopy revealed that SL and another sulfone are competitively coordinated to Li+ ions to dissociate LiPF6 in the ternary mixtures. Although the ionic conductivity decreased with increasing LiPF6 concentration due to an increase in viscosity, Li+ ions diffused faster than PF6−via exchanging ligands in the HCE [LiPF6]/[SL]/[DMS] = 1/2/2, resulting in a higher Li ion transference number than that in conventional Li battery electrolytes.


 Please wait while we load your content...
Please wait while we load your content...