A Maxwell relation for dynamical timescales with application to the pressure and temperature dependence of water self-diffusion and shear viscosity
Abstract
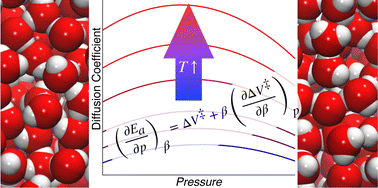
A Maxwell relation for a reaction rate constant (or other dynamical timescale) obtained under constant pressure, p, and temperature, T, is introduced and discussed. Examination of this relationship in the context of fluctuation theory provides insight into the p and T dependence of the timescale and the underlying molecular origins. This Maxwell relation motivates a suggestion for the general form of the timescale as a function of pressure and temperature. This is illustrated by accurately fitting simulation results and existing experimental data on the self-diffusion coefficient and shear viscosity of liquid water. A key advantage of this approach is that each fitting parameter is physically meaningful.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...