Triisobutylaluminium-promoted formation of lanthanide hydrides†
Abstract
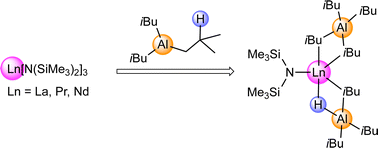
Discrete lanthanide(III) isobutylaluminates Ln[N(SiMe3)2](HAliBu3)(AliBu4) (Ln = La, Pr, Nd) are obtained from Ln[N(SiMe3)2]3 and triisobutylaluminium (TIBA). Nd[N(SiMe3)2](HAliBu3)(AliBu4) reacts with crown ether to give the ion pair [Nd(18-c-6){N(SiMe3)2}(HAliBu3)][AliBu4], featuring a strong Nd–H interaction in the solid state. The equimolar reaction of La[N(SiMe3)2](HAliBu3)(AliBu4) with fluorene resulted in the concomitant formation of [(μ-fluorenyl)3La2(μ-H)(HAliBu3)2] and (fluorenyl)2La[N(SiMe3)2]. [(μ-Fluorenyl)3La2(μ-H)(HAliBu3)2] features fluorenyl ligands with a μ-η6:η6 coordination around the hydrido-bridged dilanthanum core motif. The reported complexes are the first crystallographically characterized, ancillary ligand-free lanthanide(III) tetraisobutylaluminates, and display potential model systems for Ziegler-type polymerization catalysis.


 Please wait while we load your content...
Please wait while we load your content...