Abstract
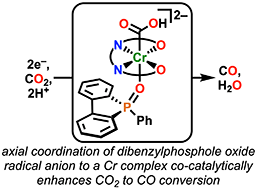
We report a co-electrocatalytic system for the selective reduction of CO2 to CO, comprised of a previously reported molecular Cr complex and 5-phenylbenzo[b]phosphindole-5-oxide (PhBPO) as a redox mediator. Under protic conditions, the co-electrocatalytic system attains a turnover frequency (TOF) of 15 s−1 and quantitative selectivity for CO. It is proposed that PhBPO interacts with the Cr-based catalyst, coordinating in an axial position trans to an intermediate hydroxycarbonyl species, M–CO2H, mediating electron transfer to the catalyst and lowering the barrier for C–OH bond cleavage.


 Please wait while we load your content...
Please wait while we load your content...