Oxoammonium salt-promoted diverse functionalization of saturated cyclic amines with dinucleophiles†
Abstract
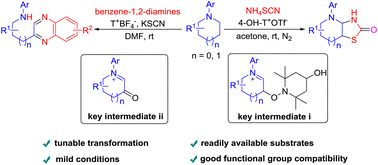
Oxoammonium salt-promoted diverse functionalization of saturated cyclic amines with different dinucleophiles under mild conditions is presented. Specifically, when thiocyanate is used as a 1,3-dinucleophile, hexahydrothiazolo[4,5-b]pyridin-2(3H)-one derivatives are formed via the formation of the β-TEMPO-tethered cyclic iminium ion as a key intermediate. By contrast, when benzene-1,2-diamine is used as a 1,4-dinucleophile, 2-alkylquinoxaline derivatives are afforded via generation of the β-oxo cyclic iminium ion as a key intermediate. In addition, the usefulness of 2-alkylquinoxalines is showcased through their facile conversion into N-(2-oxo-2-(quinoxalin-2-yl)ethyl)nitrous amides featuring the synthetically useful N–NO moiety and the carbonyl group.


 Please wait while we load your content...
Please wait while we load your content...