Topotactic redox cycling in SrFeO2.5+δ explored by 3D electron diffraction in different gas atmospheres†
Abstract
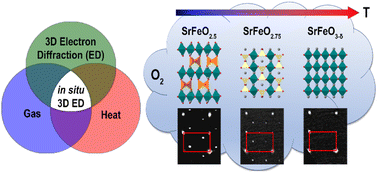
For oxygen conducting materials applied in solid oxide fuel cells and chemical-looping processes, the understanding of the oxygen diffusion mechanism and the materials' crystal structure at different stages of the redox reactions is a key parameter to control their performance. In this paper we report the first ever in situ 3D electron diffraction (ED) experiment in a gas environment and with it uncover the structure evolution of SrFeO2.5 as notably different from that reported from in situ X-ray and in situ neutron powder diffraction studies in gas environments. Using in situ 3D ED on submicron sized single crystals, we observe the transformation under O2 flow of brownmillerite SrFeO2.5 with an intra- and interlayer ordering of the left and right twisted (FeO4)∞ tetrahedral chains (space group Pcmb) into consecutively SrFeO2.75 with space group Cmmm (at 350 °C, 33% O2) and SrFeO3−δ with space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (at 400 °C, 100% O2). Upon reduction in H2 flow, the crystals return to the brownmillerite structure with intralayer order, but without regaining the interlayer order of the pristine crystals. Therefore, redox cycling of SrFeO2.5 crystals in O2 and H2 introduces stacking faults into the structure, resulting in an I2/m(0βγ)0s symmetry with variable β.
m (at 400 °C, 100% O2). Upon reduction in H2 flow, the crystals return to the brownmillerite structure with intralayer order, but without regaining the interlayer order of the pristine crystals. Therefore, redox cycling of SrFeO2.5 crystals in O2 and H2 introduces stacking faults into the structure, resulting in an I2/m(0βγ)0s symmetry with variable β.


 Please wait while we load your content...
Please wait while we load your content...