Regioselectivity of concerted proton–electron transfer at the surface of a polyoxovanadate cluster†
Abstract
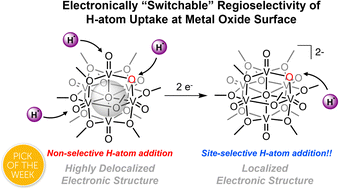
Proton-coupled electron transfer (PCET) is an important process in the activation and reactivity of metal oxide surfaces. In this work, we study the electronic structure of a reduced polyoxovanadate-alkoxide cluster bearing a single bridging oxide moiety. The structural and electronic implications of the incorporation of bridging oxide sites are revealed, most notably resulting in the quenching of cluster-wide electron delocalization in the most reduced state of the molecule. We correlate this attribute to a change in regioselectivity of PCET to the cluster surface (e.g. reactivity at terminal vs. bridging oxide groups). Reactivity localized at the bridging oxide site enables reversible storage of a single H-atom equivalent, changing the stoichiometry of PCET from a 2e−/2H+ process. Kinetic investigations indicate that the change in site of reactivity translates to an accelerated rate of e−/H+ transfer to the cluster surface. Our work summarizes the role which electronic occupancy and ligand density play in the uptake of e−/H+ pairs at metal oxide surfaces, providing design criteria for functional materials for energy storage and conversion processes.
- This article is part of the themed collections: Most popular 2023 inorganic chemistry articles, 2023 Chemical Science HOT Article Collection and 2023 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...