Chiral phosphoric acid-catalyzed Friedel–Crafts reaction of 2,5-disubstituted and 2-monosubstituted pyrroles with isoindolinone-derived ketimines†
Abstract
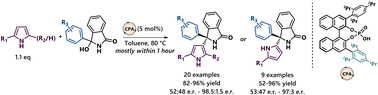
The enantioselective reaction between 2,5-disubstituted pyrroles and diaryl-ketimines, generated in situ from isoindolinone-derived alcohols, is described. Pyrrole derivatives possessing a congested tetrasubstituted stereogenic center at the β-(C3) position are generally obtained in high yields and enantioselectivities. The transformation can be extended to 2-monosubstituted pyrroles, generating chiral α-(C5) functionalized pyrrole products. Control experiments were conducted in order to elucidate the origin of the low enantioselectivities observed in some of the products.


 Please wait while we load your content...
Please wait while we load your content...