Synthesis, conformational stability and molecular structure of 4-aryl- and 4,5-diaryl-1,8-bis(dimethylamino)naphthalenes†
Abstract
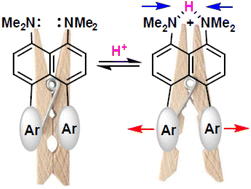
4-Bromo- and 4,5-dibromo-1,8-bis(dimethylamino)naphthalenes were arylated with arylboronic acids under Suzuki reaction conditions to provide 4-aryl- and 4,5-diaryl-1,8-bis(dimethylamino)naphthalenes, respectively. The interaction of 4,5-dibromo-1,8-bis(dimethylamino)naphthalene with pyridin-3-ylboronic acid was accompanied by heterocyclization leading unexpectedly to the formation of N3,N3,N4,N4-tetramethylacenaphtho[1,2-b]pyridine-3,4-diamine. Dynamic 1H NMR experiments showed fast interconversion between syn and anti conformers of 4,5-diaryl-1,8-bis(dimethylamino)naphthalenes in CDCl3 solution at room temperature. The free energy of the rotational isomerization was determined to be ∼14.0 kcal mol−1 for 4,5-di(m-tolyl) and 4,5-di(naphthalen-2-yl) derivatives. X-ray analysis of 4,5-diaryl-1,8-bis(dimethylamino)naphthalenes revealed a high degree of structural deformation due to internal steric repulsions between both peri-dimethylamino and peri-aryl groups. In crystals, 4,5-di(naphthalen-1-yl)-1,8-bis(dimethylamino)naphthalene molecules exist exclusively in the most stable anti-out form, while for 4,5-di(naphthalen-2-yl) and 4,5-di(m-tolyl) counterparts, only the syn-form is realized. The introduction of two peri-aryl substituents in the 1,8-bis(dimethylamino)naphthalene scaffold affected the basic properties, making the 4,5-diphenyl derivative 0.7 pKa units less basic. The protonation of 4,5-diaryl-1,8-bis(dimethylamino)naphthalenes leads to dramatic changes in their structures. Compared to the corresponding bases, the inter-nitrogen distance in these salts noticeably decreases whereas peri-aromatic rings move away from each other demonstrating the so-called “clothespin effect”. This lowers the barriers of syn/anti-isomerization; as a result, protonated molecules with peri-m-tolyl and even peri-(naphthalen-2-yl) substituents exist in crystals as mixtures of rotamers.


 Please wait while we load your content...
Please wait while we load your content...