Organocatalytic asymmetric synthesis of oxazolidino spiropyrazolinones via N,O-acetalization/aza Michael addition domino reaction between N-Boc pyrazolinone ketimines and γ-hydroxyenones†
Abstract
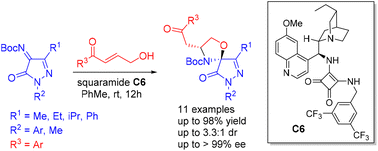
A squaramide-catalyzed asymmetric N,O-acetalization/aza Michael addition domino reaction between N-Boc ketimines derived from pyrazolin-5-ones and γ-hydroxyenones has been developed for the construction of pyrazolinone embedded spirooxazolidines. A hydroquinine derived bifunctional squaramide catalyst was found to be the most effective for this cascade spiroannulation. This new protocol allows the generation of two stereocenters and the desired products are obtained in good yields with moderate to good diastereoselectivities (up to 3.3 : 1 dr) and high enantioselectivities (up to >99% ee) from a range of substituted N-Boc pyrazolinone ketimines and γ-hydroxyenones. The developed protocol is amenable for a scale-up reaction.


 Please wait while we load your content...
Please wait while we load your content...