Catalyst-free thiophosphorylation of in situ formed ortho-quinone methides†
Abstract
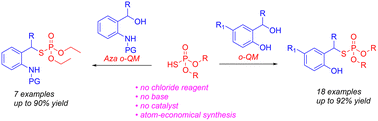
A metal-, chloride reagent and base-free thiophosphorylation reaction of in situ formed ortho-quinone methide (o-QM) to synthesize functionalized thiophosphates has been developed. The reaction is an atom-economical process, producing water as the sole byproduct. (EtO)2P(O)SH functions as both a Brønsted acid and nucleophilic thiolate to produce the o-QM intermediate and the thiophosphate product, respectively. The aza o-QMs were also successfully thiophosphorylated in the presence of catalytic TsOH to form sulfonamido thiophosphates.


 Please wait while we load your content...
Please wait while we load your content...