Water-soluble thienoviologen derivatives for imaging bacteria and antimicrobial photodynamic therapy†
Abstract
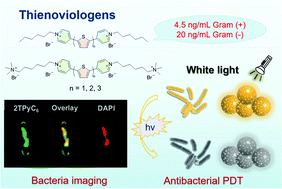
A series of water-soluble cationic thienoviologen derivative photosensitizers (nTPy-Rs) for photodynamic therapy (PDT) is reported. Cationic pyridine groups were introduced into the thiophene framework to enhance solubility and bacteria-binding ability, which effectively improved bacteriological imaging and antibacterial activity. The optoelectronic properties of nTPy-Rs were regulated by adjusting the number of thiophene groups, and the differences in antibacterial activity due to the functional scaffolds were compared. The results showed that nTPy-Rs could generate reactive oxygen species (ROS, including macroscopic free radicals), efficiently inhibit bacterial growth, and achieve the minimum inhibitory concentration (MIC) to the ng mL−1 level. Remarkably, 2TPyC6, containing two thiophene groups and modified by alkyl side chains, showed the best bacteriostatic performance, with the MIC of 20 ng mL−1 and 4.5 ng mL−1 for E. coli and S. aureus, respectively, which are the lowest photosensitizer concentrations used in PDT to date. The low cell cytotoxicity and excellent antibacterial performance give nTPy-Rs great potential as PDT agents in vivo.


 Please wait while we load your content...
Please wait while we load your content...