Platinum-complexed phosphorous-doped carbon nitride for electrocatalytic hydrogen evolution†
Abstract
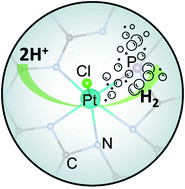
Sustainable hydrogen gas production is critical for future fuel infrastructure. Here, a series of phosphorous-doped carbon nitride materials were synthesized by thermal annealing of urea and ammonium hexafluorophosphate, and platinum was atomically dispersed within the structural scaffold by thermal refluxing with Zeise's salt forming Pt–N/P/Cl coordination interactions, as manifested in X-ray photoelectron and absorption spectroscopic measurements. The resulting materials were found to exhibit markedly enhanced electrocatalytic activity towards the hydrogen evolution reaction (HER) in acidic media, as compared to the P-free counterpart. This was accounted for by P doping that led to a significantly improved charge carrier density within C3N4, and the sample with the optimal P content showed an overpotential of only −22 mV to reach the current density of 10 mA cm−2, lower than that of commercial Pt/C (−26 mV), and a mass activity (7.1 mA μg−1Pt at −70 mV vs. reversible hydrogen electrode) nearly triple that of the latter. Results from the present study highlight the significance of P doping in the manipulation of the electronic structures of metal/carbon nitride nanocomposites for high-performance HER electrocatalysis.
- This article is part of the themed collections: Editor’s Choice 2023: Advancing electrocatalysts for a sustainable future. and Single-Atom Catalysis


 Please wait while we load your content...
Please wait while we load your content...