Na2.4Al0.4Mn2.6O7 anionic redox cathode material for sodium-ion batteries – a combined experimental and theoretical approach to elucidate its charge storage mechanism†
Abstract
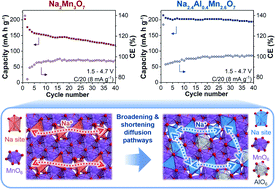
Here we report the synthesis via ceramic methods of the high-performance Mn-rich Na2.4Al0.4Mn2.6O7 oxygen-redox cathode material for Na-ion batteries, which we use as a testbed material to study the effects of Al substitution and subsequent Na excess in the high-capacity, anionic redox-based cathode material, Na2Mn3O7. The material shows a stable electrochemical performance, with a specific capacity of 215 mA h g−1 in the 1.5–4.7 V voltage range at C/20 and a capacity retention of 90% after 40 cycles. Using a combination of electrochemical and structural analysis together with hybrid density functional theory calculations we explain the behaviour of this material with changes in Mn/anionic redox reactions and associated O2 release reactions occurring during electrochemical cycling (Na+ ion insertion/extraction), and compare these findings to Na2Mn3O7. We expect that these results will advance understanding of the effect of dopants in Mn-rich cathode materials with oxygen redox activity to pave their way towards high-performance sodium-ion batteries.
- This article is part of the themed collections: 2022 Journal of Materials Chemistry Lectureship shortlisted candidates and Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...