Covalent organic frameworks anchored with frustrated Lewis pairs for hydrogenation of alkynes with H2†
Abstract
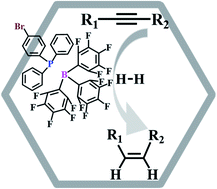
Frustrated Lewis Pair (FLP) chemistry has been widely explored in the field of catalytic hydrogenation. However, FLPs, which are usually used as homogeneous catalysts, quickly lose their catalytic reactivity in the process of recycling due to their solubility and instability. Herein, we provide a solution to this issue by anchoring triaryl phosphorus used as the Lewis base of FLPs in two kinds of bromine functionalized covalent organic frameworks (COFs). Then, tris(pentafluorophenyl)boron (BCF) was introduced as the Lewis acid of FLPs, resulting in COF supported heterogeneous FLP catalysts, called COFs-FLPs which are the first COFs anchored with FLPs. These heterogeneous COFs-FLP catalysts show excellent catalytic reactivity for the hydrogenation of alkynes to olefins. More importantly, these COFs-FLPs can be easily re-cycled at least 10 times without losing their catalytic reactivity.


 Please wait while we load your content...
Please wait while we load your content...