Increasing H2 production on ZnxInySz under visible light via composition modulation and CoS catalysis†
Abstract
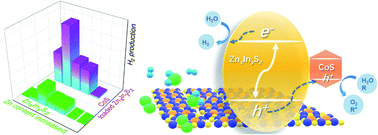
Zinc indium sulfide materials (ZnxIn2Sx+3, x = 1–5) are known to initiate H2 production under visible light, among which ZnIn2S4 is the most active with triethanolamine (TEOA) as the sacrificial agent. Herein a new series of ZIS (ZnIn6S10, ZnIn2S4, Zn4In2S7, Zn6In2S9, and Zn11In2S14) have been synthesized via a hydrothermal method. For H2 production in the presence of TEOA under a 420 nm LED lamp, Zn6In2S9 was not only the most active among the ZIS materials, but also much more active than ZnS, In2S3, and CdS. With the increase of the Zn content, ZIS had an increased band gap, but a negatively shifted flat band potential in aqueous solution. Because of that, Zn6In2S9 was more active than others. Furthermore, after 20 wt% CoS loading, ZIS had a 2.9–6.8 fold increased activity, in terms of the amount of H2 evolved in 2 h. Through a measurement of proton reduction, open circuit potential, and charge transfer resistance, a possible mechanism is proposed, involving hole transfer from ZIS to CoS, followed by increased TEOA oxidation and proton reduction. This work provides a simple way to modulate the performance of metal chalcogenides for water splitting under visible light.

 Please wait while we load your content...
Please wait while we load your content...