Co3Mo3N as an alternative for noble-metal catalysts in hydrodeoxygenation of methyl palmitate to diesel range hydrocarbons†
Abstract
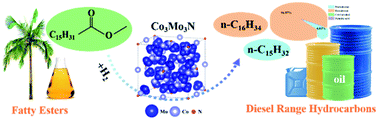
Hydrodeoxygenation (HDO) of fatty triglycerides into diesel range hydrocarbons over non-noble-metal catalysts is highly desirable for effectively solving the problems of energy depletion and carbon emission. In this study, the most efficient catalytic system (Co3Mo3N) achieves almost complete conversion of methyl palmitate (MPA, a typical ester exchange product of palmitic acid triglyceride and methanol) (99.5%) with 95.0% selectivity to hexadecane under mild conditions, which is attributed to the synergistic interactions between Co and Mo nitrides, forming a unique dual-site configuration of the Co3Mo3N catalyst. The reaction kinetics analysis shows that HDO instead of decarboxylation/decarbonylation is the primary reaction route for the hydrogenation of MPA over the Co3Mo3N catalyst. Significantly, Co3Mo3N exhibits good stability for 72 h. These results suggest that the highly efficient cobalt-molybdenum bimetallic catalyst with an optimized fine structure and electronic coordination environment could achieve the production of clean fuels from the HDO of fatty acids/esters.


 Please wait while we load your content...
Please wait while we load your content...