Ten-step asymmetric total syntheses of potent antibiotics anthracimycin and anthracimycin B†
Abstract
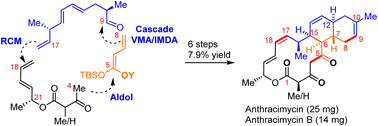
The increase in antibiotic resistance calls for the development of novel antibiotics with new molecular structures and new modes of action. However, in the past few decades only a few novel antibiotics have been discovered and progressed into clinically used drugs. The discovery of a potent anthracimycin antibiotic represents a major advance in the field of antibiotics. Anthracimycin is a structurally novel macrolide natural product with an excellent biological activity profile: (i) potent in vitro antibacterial activity (MIC 0.03–1.0 μg mL−1) against many methicillin-resistant Staphylococcus aureus (MRSA) strains, Bacillus anthracis (anthrax), and Mycobacterium tuberculosis; (ii) low toxicity to human cells (IC50 > 30 μM); (iii) a novel mechanism of action (inhibiting DNA/RNA synthesis). While the first total synthesis of anthracimycin was elegantly accomplished by Brimble et al. with 20 steps, we report a 10-step asymmetric total synthesis of anthracimycin and anthracimycin B (first total synthesis). Our convergent strategy features (i) one-pot sequential Mukaiyama vinylogous aldol/intramolecular Diels–Alder reaction to construct trans-decalin with high yield and excellent endo/exo selectivity and (ii) Z-selective ring-closing metathesis to forge the 14-membered ring. In vitro antibacterial evaluation suggested that our synthetic samples exhibited similar antibacterial potency to the naturally occurring anthracimycins against Gram-positive strains. Our short and reliable synthetic route provides a supply of anthracimycins for further in-depth studies and allows medicinal chemists to prepare a library of analogues for establishing structure–activity relationships.


 Please wait while we load your content...
Please wait while we load your content...