Phosphine-catalyzed activation of cyclopropenones: a versatile C3 synthon for (3+2) annulations with unsaturated electrophiles†
Abstract
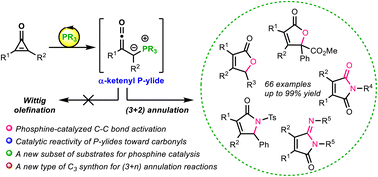
We herein report a phosphine-catalyzed (3 + 2) annulation of cyclopropenones with a wide variety of electrophilic π systems, including aldehydes, ketoesters, imines, isocyanates, and carbodiimides, offering products of butenolides, butyrolactams, maleimides, and iminomaleimides, respectively, in high yields with broad substrate scope. An α-ketenyl phosphorous ylide is validated as the key intermediate, which undergoes preferential catalytic cyclization with aldehydes rather than stoichiometric Wittig olefinations. This phosphine-catalyzed activation of cyclopropenones thus supplies a versatile C3 synthon for formal cycloadditon reactions.


 Please wait while we load your content...
Please wait while we load your content...