Ligand-promoted palladium-catalyzed β-methylene C–H arylation of primary aldehydes†
Abstract
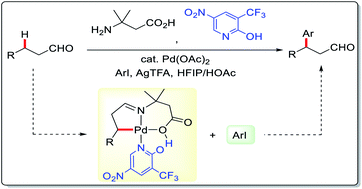
The transient directing group (TDG) strategy allowed long awaited access to the direct β-C(sp3)–H functionalization of unmasked aliphatic aldehydes via palladium catalysis. However, the current techniques are restricted to terminal methyl functionalization, limiting their structural scopes and applicability. Herein, we report the development of a direct Pd-catalyzed methylene β-C–H arylation of linear unmasked aldehydes by using 3-amino-3-methylbutanoic acid as a TDG and 2-pyridone as an external ligand. Density functional theory calculations provided insights into the reaction mechanism and shed light on the roles of the external and transient directing ligands in the catalytic transformation.


 Please wait while we load your content...
Please wait while we load your content...