Diffusion of dyes in polyelectrolyte-surfactant hydrogels
Abstract
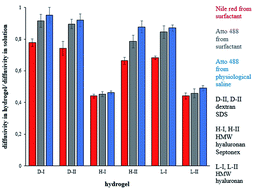
In this work, hydrogels formed by interaction of biopolymeric electrolytes and oppositely charged surfactants are studied from the point of view of their ability to incorporate model hydrophobic dyes in their micelle-like structure. Two types of hydrogels were investigated. The first type was based on cationized dextran cross-linked by sodium dodecylsulphate. The second type was prepared by interactions of hyaluronan with carbethoxypendecinium bromide (septonex). Nile red and Atto488 were used as model dyes for the diffusion experiments. The dyes were dissolved in two different media: surfactant and physiological saline. The diffusion of dyes into hydrogel was monitored over time. Effective diffusion coefficients were determined. It was found that their values are strongly influenced by the hydrogel character, the types of dye used and the solvent. The obtained effective coefficients were higher in comparison with the values determined for the diffusion in the opposite direction (release from the hydrogel). The dyes are presented as free in physiological saline and in the form of micelles or micelle aggregates in surfactants. During diffusion into the hydrogel, they can be gradually incorporated in a “pearl necklace structure” which suppresses their mobility. In contrast, this partial immobilization of dyes can increase the concentration gradient which is a driving force of diffusion. Also, the gradual incorporation of dyes into hydrogel structures influences the values of the effective diffusion coefficients.


 Please wait while we load your content...
Please wait while we load your content...