Proton reduction by a bimetallic zinc selenolate electrocatalyst†
Abstract
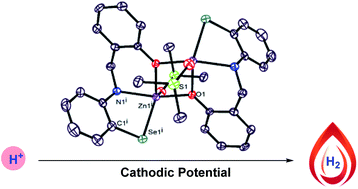
The development of alternative energy sources is the utmost priority of developing society. Unlike many prior homogeneous electrocatalysts that rely on a change in the oxidation state of the metal center and/or electrochemically active ligand, here we report the synthesis and structural characterization of a bimetallic zinc selenolate complex consisting of a redox silent zinc metal ion and a tridentate ligand that catalyzes the reduction of protons into hydrogen gas electrochemically and displays one of the highest reported TOF for a homogeneous TM-metal free ligand centered HER catalyst, 509 s−1. The current–voltage analysis confirms the onset overpotential of 0.86 V vs. Ag/AgCl for the HER process. Constant potential electrolysis (CPE) has been carried out to study the bulk electrolysis of our developed protocol, which reveals that the bimetallic zinc selenolate catalyst is stable under cathodic as well as anodic potentials and generates hydrogen gas with a faradaic efficiency of 75%. Preliminary studies on the heterogeneous catalyst were conducted by depositing the bimetallic zinc selenolate catalyst on the electrode surface.


 Please wait while we load your content...
Please wait while we load your content...