Design and synthesis of amino acid derivatives of substituted benzimidazoles and pyrazoles as Sirt1 inhibitors†
Abstract
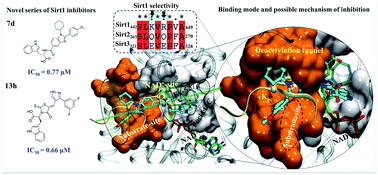
Owing to its presence in several biological processes, Sirt1 acts as a potential therapeutic target for many diseases. Here, we report the structure-based designing and synthesis of two distinct series of novel Sirt1 inhibitors, benzimidazole mono-peptides and amino-acid derived 5-pyrazolyl methylidene rhodanine carboxylic acid. The compounds were evaluated for in vitro enzyme-based and cell-based Sirt1 inhibition assay, and cytotoxic-activity in both liver and breast cancer cells. The tryptophan conjugates i.e. 13h (IC50 = 0.66 μM, ΔGbind = −1.1 kcal mol−1) and 7d (IC50 = 0.77 μM, ΔGbind = −4.4 kcal mol−1) demonstrated the maximum efficacy to inhibit Sirt1. The MD simulation unveiled that electrostatic complementarity at the substrate-binding-site through a novel motif “SLxVxP(V/F)A” could be a cause of increased Sirt1 inhibition by 13h and 13l over Sirt2 in cell-based assay, as compared to the control Ex527 and 7d. Finally, this study highlights novel molecules 7d and 13h, along with a new key hot-spot in Sirt1, which could be used as a starting lead to design more potent and selective sirtuin inhibitors as a potential anticancer molecule.
- This article is part of the themed collection: 2022 RSC Advances Popular Advances Collection


 Please wait while we load your content...
Please wait while we load your content...