Transition-metal-free, visible-light-induced multicomponent synthesis of allylic amines and tetrahydroquinolines†
Abstract
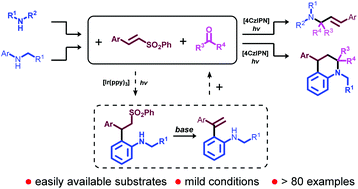
A visible-light-induced, 1,2,3,5-tetrakis-(carbazolyl)-4,6-dicyanobenzene (4CzIPN) catalyzed synthesis of allylic amines from secondary alkylamines, alkyl-substituted carbonyls, and vinyl sulfones through ‘all-alkyl’ α-amino radicals has been developed. In another similar photoreductive system, 4CzIPN catalyzed the synthesis of tetrahydroquinolines from 2-vinylanilines and alkyl-substituted carbonyls through anilinoalkyl radicals. Both reactions exhibit a good substrate scope and functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...