Hyperispirones A and B, spiro-bridged polycyclic polyprenylated acylphloroglucinols with anti-angiogenesis activity from Hypericum beanii†
Abstract
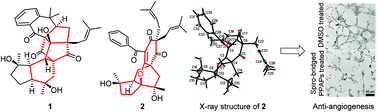
Two unprecedented spiro-bridged polycyclic polyprenylated acylphloroglucinols (PPAPs), one with a caged heptacyclo[18.3.1.01,20.02,17.03,15.06,15.08,13]tetracosane core bearing a dual-bridged spiro central feature (1) and the other with an unusual 8-oxa-tetracyclo[7.4.2.01,9,03,7]cetane ring system (2), were isolated and characterized from the aerial parts of Hypericum beanii. Their structures were elucidated by extensive analysis of HRESIMS, NMR, quantum-chemical calculations, and X-ray crystallographic data. A putative biosynthetic pathway for 1 was also proposed. Compounds 1 and 2 exhibited significant angiogenesis inhibitory effects against the tube formation of HUVECs.


 Please wait while we load your content...
Please wait while we load your content...