Thioether analogues of the pituitary neuropeptide oxytocin via thiol–ene macrocyclisation of unprotected peptides†
Abstract
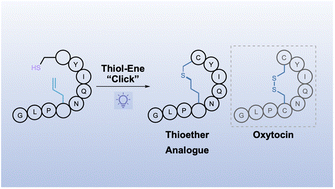
Disulfide bonds are an essential feature of many bioactive peptides, however, they are labile to reducing conditions which can limit therapeutic application. Herein, we report an efficient methodology for peptide macrocyclisation, furnishing thioether mimetics of disulfide linkages via thiol–ene click chemistry. Furthermore, this methodology is applied to the efficient synthesis of analogues of the neuropeptide oxytocin and in a highly efficient route to the clinical therapeutic carbetocin.


 Please wait while we load your content...
Please wait while we load your content...