Facile construction of C,N-disulfonated 5-amino pyrazoles through an iodine-catalyzed cascade reaction†
Abstract
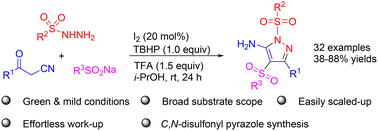
A green and facile synthesis of previously unreported C,N-disulfonated 5-amino pyrazoles was established through an iodine-catalyzed cascade reaction of easily accessible sulfonyl hydrazides, β-ketonitriles, and sodium sulfinates. Diverse C,N-disulfonated 5-amino pyrazoles could be obtained in 38–88% yields. This methodology features green and mild conditions, broad substrate scope, and effortless work-up.


 Please wait while we load your content...
Please wait while we load your content...