Photoinduced rearrangement of α-(2-nitrophenyl)ketones†
Abstract
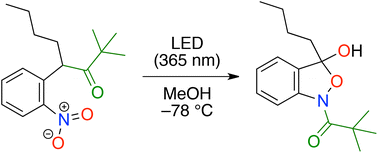
Photoirradiation of α-(2-nitrophenyl)ketones produced cyclic hydroxamates. The reaction proceeded via photoinduced oxygen transfer from the nitro group to the benzylic position, forming an α-hydroxyketone having a nitroso group. Subsequent addition of the nitroso group to the ketone moiety and the concomitant cleavage of the C–C σ bond between the carbonyl group and the benzyl position produced hydroxamic acid, which underwent formation of a hemiacetal to give cyclic hydroxamate.


 Please wait while we load your content...
Please wait while we load your content...