Efficient catalyst-free direct amidation of non-activated carboxylic acids from carbodiimides†
Abstract
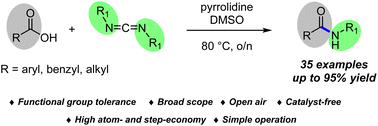
A novel and efficient catalyst- and activating agent-free amidation method via direct amidation of carboxylic acids where carbodiimides act as a reagent instead of an activating agent is reported. The reaction is conducted under non-traditional coupling conditions where a higher temperature is employed. Besides not using stoichiometric ratios of activating agent or catalyst, this approach is made even more attractive by occurring in the presence of the environmentally friendly and recyclable non-toxic solvent of DMSO. A wide variety of benzylic, aliphatic, α,β-unsaturated and aromatic carboxylic acids provide related amides in up to 95% yield. The excellent yield from a gram-scale reaction shows that this application is particularly convenient for larger-scale synthesis applications.


 Please wait while we load your content...
Please wait while we load your content...