Reductive coupling of allenoates with aldehydes catalyzed by halogenotin hydride†
Abstract
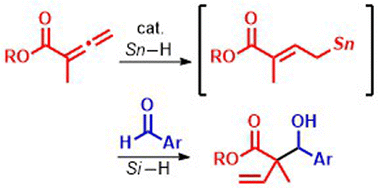
Hydrostannylation of allenoate 1 with halogenotin hydride (Bu2SnClH) was developed to give allylic stannane (I), which then could react with aldehyde 2. The same coupling gave allylic alcohol 3 under reductive conditions in the presence of a silane and a catalytic amount of Bu2SnClH. This catalytic protocol was applied to the synthesis of lactone 4. The resultant products 3 and 4 were subjected to oxidative conditions to provide oxacycles 5 and 6, respectively.


 Please wait while we load your content...
Please wait while we load your content...