Solvent-controlled base-free synthesis of bis(trifluoromethyl)-cyclopropanes and -pyrazolines via cycloaddition of 2-trifluoromethyl-1,3-enynes with 2,2,2-trifluorodiazoethane†
Abstract
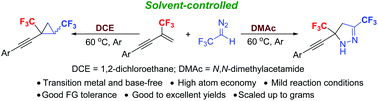
A highly efficient solvent-controlled synthesis of bis(trifluoromethyl)cyclopropanes and bis(trifluoromethyl)pyrazolines via a [2 + 1] or [3 + 2] cycloaddition reaction of 2-trifluoromethyl-1,3-conjugated enynes with CF3CHN2 was developed. The reactions of 2-trifluoromethyl-1,3-conjugated enynes with CF3CHN2 proceeded smoothly under transition-metal and base-free conditions, affording the expected cycloaddition products in good to excellent yields. When DMAc (N,N-dimethylacetamide) was used as the solvent, bis(trifluoromethyl)pyrazolines were obtained; however, in contrast, bis(trifluoromethyl)cyclopropanes were formed by changing the solvent from DMAc to DCE (1,2-dichloroethane).


 Please wait while we load your content...
Please wait while we load your content...