Asymmetric transfer hydrogenation of boronic acid pinacol ester (Bpin)-containing acetophenones†
Abstract
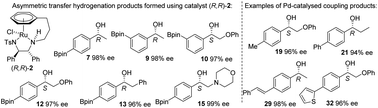
A series of Bpin-containing acetophenone derivatives were reduced by asymmetric transfer hydrogenation (ATH), using Noyori–Ikariya catalysts, with formic acid/triethylamine, to alcohols in high ee when the Bpin is in the para- or meta-position. Substrates containing ortho-Bpin groups were reduced in lower ee, with formation of a cyclic boron-containing group. The products were converted to substituted derivatives using Pd-catalysed coupling reactions. The results represent the first examples of ATH of Bpin-containing ketones.


 Please wait while we load your content...
Please wait while we load your content...