The seven-step, one-pot regioselective synthesis of biologically important 3-aryllawsones: scope and applications†
Abstract
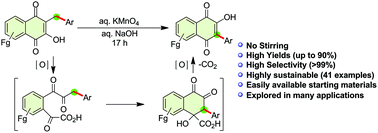
3-Aryllawsones are well known for their wide range of applications in medicinal chemistry, but their synthesis has always remained challenging as no comprehensive protocol has been outlined to date. Owing to their structural importance, we synthesized various 3-aryllawsones with high regioselectivity from simple lawsone and aldehydes in a seven-step double-cascade one-pot reaction through the combination of organocatalytic Ramachary reductive coupling and Hooker oxidation reactions. The commercial availability of the starting materials, diverse substrate scope, possibility of a one- or two-pot approach, regioselectivity of alkyl transfer (with mechanistic proof provided via X-ray crystal structure analysis), and numerous medicinal applications of 3-aryllawsones are the key attractions of this work.


 Please wait while we load your content...
Please wait while we load your content...