Nucleophilic functionalizations of indole derivatives using the aromatic Pummerer reaction†
Abstract
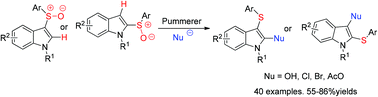
Because of the electron-rich property of indoles, direct functionalization strategies towards indoles generally involve electrophilic substitutions. In this paper, an efficient protocol for nucleophilic hydroxylation, halogenation and esterification of indoles via the aromatic Pummerer process was developed. With the advantages of readily accessible starting materials, simple operation and mild conditions, this protocol should be of interest to synthetic scientists.


 Please wait while we load your content...
Please wait while we load your content...