Co-Catalyzed atom transfer radical addition of bromodifluoroacetamides, expanding the scope of radical difluoroalkylation†
Abstract
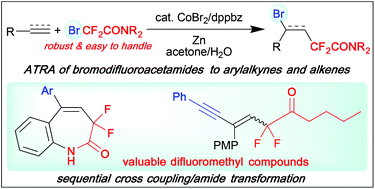
The atom transfer radical addition (ATRA) of bromodifluoroacetamides to arylalkynes and terminal alkenes was conducted using von Wangelin's Co catalyst system (CoBr2/1,2-bis(diphenylphosphino)benzene/Zn) in acetone/H2O at 30 °C to afford the corresponding functionalized difluoroacetamides in 33–89% yields. Moreover, the Co catalyst was successfully applied to the tandem addition/cyclization of 1,6-diene and -enyne substrates and intramolecular ATRA of N-allyl and N-propargyl bromodifluoroacetamides, significantly expanding the scope of radical difluoroalkylation.

 Please wait while we load your content...
Please wait while we load your content...