Cyclopenta-fused polyaromatic hydrocarbons: synthesis and characterisation of a stable, carbon-centred helical radical†
Abstract
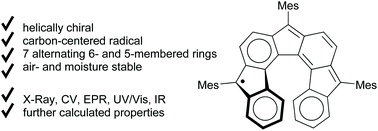
An air- and moisture-stable helical radical with seven six- and five-membered rings arranged alternately was synthesized by cyclizations in a suitably ortho,ortho′-substituted terphenyl and re-establishment of its conjugation. Mesityl groups at the five-membered rings prevent radical reactions. This cyclopenta-fused polyaromatic hydrocarbon (CP-PAH) was characterized by X-ray crystallographic analysis, EPR and UV/Vis spectroscopy, and by cyclic voltammetry. Further properties and spectra were determined by quantum chemical calculation (spin densities, orbital energies, UV/Vis/NIR and ECD spectra). It turned out that this radical is best described with its radical centre being in the outer five-membered rings, which allows for the largest number of fully intact benzene rings. Its triradical character is rather small and can be neglected. The five-membered rings show significant antiaromatic character, which is highest in the central ring.

 Please wait while we load your content...
Please wait while we load your content...