Asymmetric total synthesis of (+)-(2R,4′R,8′R)-α-tocopherol enabled by enzymatic desymmetrization†
Abstract
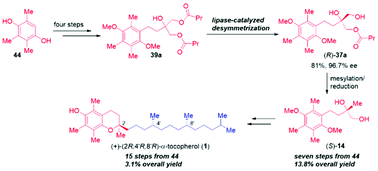
A new and highly stereoselective synthesis of chiral diol (S)-14, the common synthetic intermediate to (+)-(2R,4′R,8′R)-α-tocopherol (1), was accomplished in seven steps with 13.8% overall yield. This developed route featured a lipase-catalyzed desymmetric hydrolysis of prochiral diester 39a, which was prepared through a challenging Heck coupling, to chiral quaternary carbon-containing monoester (R)-37a of the correct configuration in 81% yield and 96.7% ee, to the best of our knowledge, leading to the most efficient enzymatic desymmetric synthesis of the chiral chroman skeleton of vitamin E members reported to date. Coupled with the modified preparation of the phytol-derived side chain, the chemoenzymatic total synthesis of 1 was completed in 15 longest linear steps with 3.1% overall yield.

 Please wait while we load your content...
Please wait while we load your content...