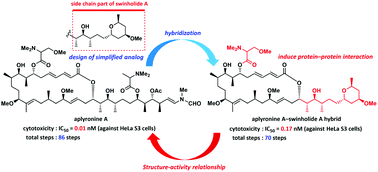
Structure–activity relationship studies on an antitumor marine macrolide using aplyronine a–swinholide A hybrid†
Abstract
An aplyronine A–swinholide A hybrid, consisting of the macrolactone part of aplyronine A and the side chain part of swinholide A, was designed, synthesized, and biologically evaluated. This hybrid induced protein–protein interactions between two major cytoskeletal proteins actin and tubulin in the same manner as aplyronine A, and exhibited potent cytotoxicity and actin-depolymerizing activity. The importance of the methoxy group in the N,N,O-trimethylserine ester was clarified by the structure–activity relationship studies of the amino acid moiety by using the hybrid analogs. Furthermore, the comparison of the actin-depolymerizing activities between the side chain analogs of aplyronine A and swinholide A showed that the side chain analog of swinholide A had much weaker actin-depolymerizing activity than that of aplyronine A.

 Please wait while we load your content...
Please wait while we load your content...