TCT-mediated click chemistry for the synthesis of nitrogen-containing functionalities: conversion of carboxylic acids to carbamides, carbamates, carbamothioates, amides and amines†
Abstract
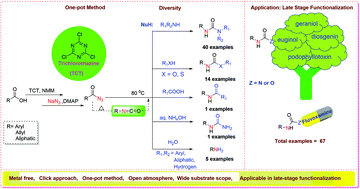
Here, we report a s-trichlorotriazine (TCT, also known as cyanuric chloride) mediated one-pot general method for the conversion of carboxylic acids into ubiquitous functionalities such as carbamides, carbamates, carbamothioates, amides, and amines. The TCT-mediated activation of acids followed by azidation and heating led to the isocyanate formation via Curtius rearrangement which involves click chemistry in the presence of nucleophiles and provided the coupled product. The TCT was employed at ≤40 mol% with respect to the starting materials; however, its bulk availability and low cost provide a unique opportunity towards its applicability in the synthesis of functional molecules. The optimized conditions have also been successfully demonstrated for gram scale synthesis and late-stage functionalization of natural products and drugs such as podophyllotoxin, eugenol, diosgenin, geraniol and fluvoxamine.


 Please wait while we load your content...
Please wait while we load your content...