A metal-free BF3·OEt2 mediated chemoselective protocol for the synthesis of propargylic cyclic imines†
Abstract
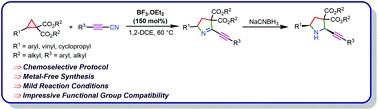
A chemoselective and metal/additive-free protocol for the synthesis of propargylic cyclic imine derivatives via (3 + 2)-cycloaddition of donor–acceptor cyclopropanes and alkynylnitriles in the presence of BF3·OEt2 has been established. The newly developed methodology provided access to a variety of propargylic cyclic imines in good to excellent yields. In addition, the synthesis of propargylic amines and the corresponding very stable enol derivatives from the title compound is also explored.


 Please wait while we load your content...
Please wait while we load your content...