Design and NMR characterization of reversible head-to-tail boronate-linked macrocyclic nucleic acids†
Abstract
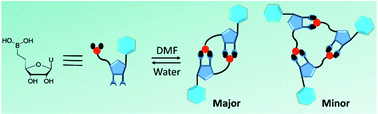
Inspired by the ability of boronic acids to bind with compounds containing diol moieties, we envisioned the formation in solution of boronate ester-based macrocycles by the head-to-tail assembly of a nucleosidic precursor that contains both a boronic acid and the natural 2′,3′-diol of ribose. DOSY NMR spectroscopy experiments in water and anhydrous DMF revealed the dynamic assembly of this precursor into dimeric and trimeric macrocycles in a concentration-dependent fashion as well as the reversibility of the self-assembly process. NMR experimental values and quantum mechanics calculations provided further insight into the sugar pucker conformation profile of these macrocycles.
- This article is part of the themed collections: Chemical Biology in OBC and Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...