Facile tin(ii)-catalyzed synthesis of N-heterocycles from dicarboxylic acids and arylamines†
Abstract
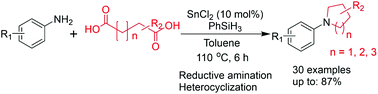
A novel efficient transformation reaction of dicarboxylic acids into N-aryl-substituted azacycles is described. In this synthetic procedure, both catalytic SnCl2 and phenylsilane were used as crucial reagents for reaction of arylamines with dicarboxylic acids to produce the desired azacycles. Using this SnCl2-catalyzed synthetic method, various N-aryl-substituted azacycles were successfully prepared from arylamines with dicarboxylic acids in high yield. This practical synthetic method using catalytic SnCl2 can provide a useful approach for preparation of the desired azacycle products from many available dicarboxylic acid starting materials.

 Please wait while we load your content...
Please wait while we load your content...