Backbone thioamide directed macrocyclisation: lactam stapling of peptides†
Abstract
A novel method for lactam stapling of Asp/Lys-containing peptides has been developed that does not require coupling agents. A backbone thioamide is incorporated at the N-terminal side of the aspartate residue. Ag(I)-promoted activation of the thioamide in the vicinity of the Asp carboxylate generates a cyclic isoimide intermediate that is trapped by the Lys amine to generate the macrolactam. This method is suitable for generation of i,i+2, i,i+3, and i,i+4-spaced lactam-bridged peptides.
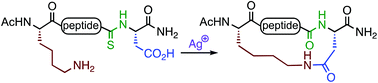


 Please wait while we load your content...
Please wait while we load your content...