Regioselective synthesis of 6-nitroindole derivatives from enaminones and nitroaromatic compounds via transition metal-free C–C and C–N bond formation†
Abstract
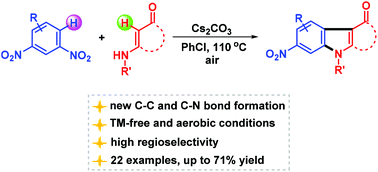
Few methods are known for the synthesis of nitroindole derivatives. A simple and practical Cs2CO3-promoted method for the synthesis of 6-nitroindole derivatives from enaminones and nitroaromatic compounds has been developed. Two new C–C and C–N bonds were formed in a highly regioselective manner under transition metal-free conditions.


 Please wait while we load your content...
Please wait while we load your content...