DABSO as a SO2 gas surrogate in the synthesis of organic structures
Abstract
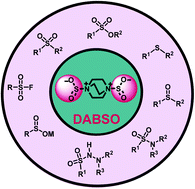
1,4-Diazabicyclo[2.2.2]octane bis(sulfur dioxide), DABCO·SO2, or DABSO, a bench-stable colorless solid, is industrially produced by the reaction of DABCO with condensed and bubbled sulfur dioxide gas at a low temperature. However, in some cases, it could catalyze organic reactions. DABSO is mostly used as a surrogate of gaseous sulfur dioxide to react with organic substrates, including Grignard reagents, aryl or alkyl halides, boronic acids, various amines, diazonium salts, carboxylic acids, heterocycles, acrylamides, alkenes, alkynes, and β-alkynyl ketones, through one-pot protocols, annulation, or coupling reactions. Most of these synthetic reactions proceed via the formation of a sulfinate radical or anion. Using DABSO as a reagent, various simple to complex structures can be constructed, such as metal sulfinates, sulfonyl fluorides, sulfonamides, sulfonohydrazides, sulfonic esters, sulfonic thioesters, and sulfones. In this review, we want to investigate mechanistically the role of DABSO in organic synthesis.


 Please wait while we load your content...
Please wait while we load your content...