Amide-derived lysine analogues as substrates and inhibitors of histone lysine methyltransferases and acetyltransferases†
Abstract
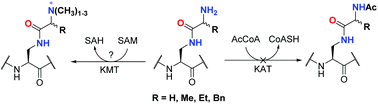
Histone lysine methyltransferases and acetyltransferases are two classes of epigenetic enzymes that play pivotal roles in human gene regulation. Although they both recognise and posttranslationally modify lysine residues in histone proteins, their difference in histone peptide-based substrates and inhibitors remains to be firmly established. Here, we have synthesised lysine mimics that posses an amide bond linker in the side chain, incorporated them into histone H3 tail peptides, and examined synthetic histone peptides as substrates and inhibitors for human lysine methyltransferases and acetyltransferases. This work demonstrates that histone lysine methyltransferases G9a and GLP do catalyse methylation of the most similar lysine mimic, whereas they typically do not tolerate more sterically demanding side chains. In contrast, histone lysine acetyltransferases GCN5 and PCAF do not catalyse acetylation of the same panel of lysine analogues. Our results also identify potent H3-based inhibitors of GLP methyltransferase, providing a basis for development of peptidomimetics for targeting KMT enzymes.


 Please wait while we load your content...
Please wait while we load your content...