Domino Michael/Michael reaction catalyzed by switchable modularly designed organocatalysts†
Abstract
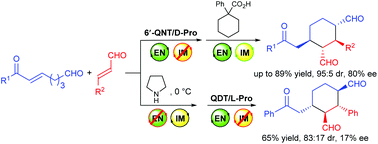
The domino Michael/Michael reaction between (E)-7-aryl-7-oxohept-5-enals and trans-cinnamaldehydes was investigated by using modularly designed organocatalysts (MDOs). It was found that both the enamine and iminium catalytic modes of the MDOs are switchable and can be individually switched on and off by using appropriate combinations of the precatalyst modules and the reaction conditions. When both the enamine and iminium catalysis modes of the MDOs are switched on, the desired domino reaction products can be obtained in good yields and stereoselectivities under optimized conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...