An exchange interaction of the antiferromagnetic nature in benzoate bridged Mn(ii) chains†
Abstract
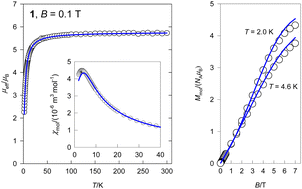
Two novel 1D chains based on heptacoordinated Mn(II) ions bridged by variously derived benzoate ligands were prepared. The unusual structures of [MnII(L1)(L2)4]n1 and [MnII(L1)(L3)4]n2 (where L1 = 2,6-dimethanolpyridine, L2 = 3,5-dinitrobenzoic acid, L3 = 4-chloro-3,5-dinitrobenzoic acid) were confirmed by single-crystal X-ray diffraction. Within the structures a zig zag pattern appears from perpendicularly located 2,6-dimethanolpyridines to sheets of benzoic acids. Both compounds possess the {MnNO6} chromophore and show paramagnetic behaviour with an exchange interaction of antiferromagnetic nature. Weak interaction constants J/hc = −0.54 cm−1 (1) and J/hc = −0.58 cm−1 (2) between high spin (S = 5/2) magnetic centres have been revealed using a finite-ring Hamiltonian model.


 Please wait while we load your content...
Please wait while we load your content...