Nickel hexacyanoferrate/graphene thin film: a candidate for the cathode in aqueous metal-ion batteries†
Abstract
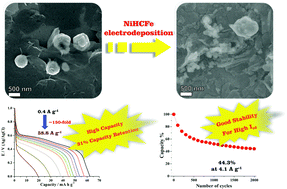
A thin film of reduced graphene oxide (rGO) and nickel nanoparticle nanocomposite was used as a precursor to synthesize a novel graphene/nickel hexacyanoferrate (rGO/NiHCFe) thin film nanocomposite through a heterogeneous electrochemical reaction with ferricyanide ions in solution. The rGO/NiHCFe thin film was morphologically, structurally and electrochemically characterized by several techniques, evidencing the intimate contact between the components characterized by a homogeneous distribution of NiHCFe nanoparticles (20 to 90 nm) over the rGO sheets. The electrochemical response of the rGO/NiHCFe-based electrodes was studied by cyclic voltammetry and charge/discharge curves in aqueous solutions of Li+, Na+ or K+. Specific capacities of up to 62.3 and 68.6 mA h g−1 (at 0.4 A g−1), and retention values of 44.3 and 49.4% (2000 cycles at 4.1 A g−1) for K+ and Na+, respectively, were found, indicating that the rGO/NiHCFe thin film is a promising candidate to be used as cathodes in K- or Na-ion aqueous batteries.


 Please wait while we load your content...
Please wait while we load your content...